Santa Rosa Junior College Student Health Services and Santa Rosa Intercultural Center co-sponsored a COVID-19 vaccine Q&A webinar with multicultural medical professionals for students and faculty Sept. 23.
Director of Student Health Services Dr. Rebecca Norwick hosted the webinar. She was joined by Dr. Kismet Baldwin, the county’s deputy public health officer; Dr. Brian Prystowsky, a Sutter Pediatrics pediatrician and a Family Medicine Residency Training Program teacher; and Dr. De Von Jackson, a primary care physician at Santa Rosa Community Health.
Q: If I already had COVID-19, would I have developed enough natural immunity that I don’t need the vaccine?
Baldwin said antibodies developed by the immune response from getting COVID-19 last for at least 90 days and possibly up to five months.
“We are recommending that you get the COVID vaccine even if you’ve had COVID. It shouldn’t hurt, and it will just boost the immune response you started out with when you got sick with COVID,” Baldwin said.
Pystowski said data from Israel suggests that some of the people with the highest immunity to COVID-19 are those who previously got COVID-19 and were vaccinated.
Q: It seems like the vaccine was developed too fast. How can I know it’s safe?
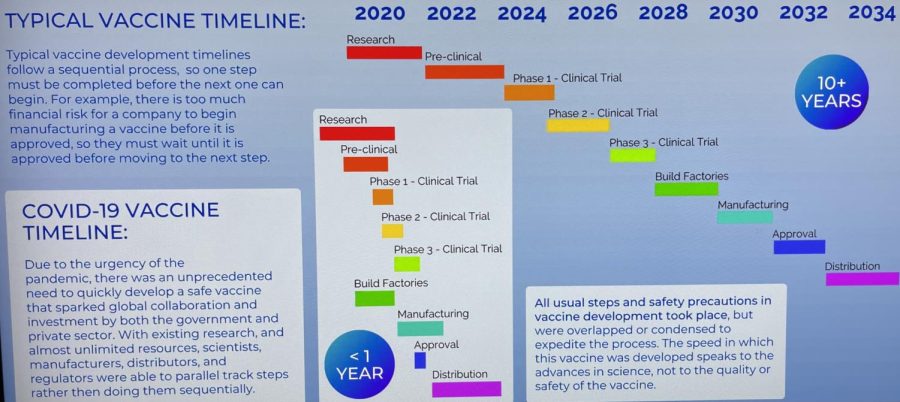
Jackson said the code name for COVID-19 virus is SARS-CoV-2, which stands for Severe Acute Respiratory Syndrome Coronavirus 2. The process to create the vaccine began when Coronavirus 1 showed up more than 10 years ago, but halted just before completion when funding ran out.
“The research was not ‘thrown together,’” Jackson said. “Some people describe it as a car that has been completely prepared on the assembly line. It’s just missing the tires. So very little had to be added. We just put the tires on.”
Baldwin said the COVID-19 vaccine went through the same rigorous process as previous vaccines.
Q: What about the long-term effects? What happens in three, five and 10 years?
Prystowsky said people might think the vaccine hasn’t been around long enough to know the long-term side effects, but typically with vaccine research, long-term effects are seen within 60 days of the phase 3 trials. He said this is what Center for Disease Control national researchers found for all 14 vaccines used in the U.S. Since the phase 3 trial for the COVID-19 vaccine was conducted from March-September 2020 and millions of people have received the vaccine so far, Prystowsky thinks we would have seen long-term effects by now.
Prystowsky said COVID-19 is less predictable and it’s hard to know who is or isn’t going to have a severe reaction to the disease.
“I can say as a pediatrician, we don’t know which children in a post-COVID infection might have multi-organ inflammatory syndrome, or MISC,” Prystowski said. “It’s not clear what the initial severity of the infection has to be in order to be subject to those long-term unpredictable inflammatory sequelae from a COVID infection.”
Q: What is FDA approval?
Baldwin said FDA approval is a process to determine if the vaccine is safe and effective. All of the vaccines were able to get emergency use authorization for the pandemic from the FDA before approval because each company completed its trial periods. To get full FDA approval, the FDA must watch the data to determine the vaccine’s safety and efficacy based on real world results. So far Pfizer has gotten approval for people 17 and up, and Dr. Baldwin expects the same approval will soon happen for the Moderna and Johnson & Johnson vaccines.
Q: What about people who have died from getting the vaccine?
Prystowsky said there were cases of people getting blood clots from receiving the J&J vaccine, but this is the base of the risk calculation we need to consider. The news of the blood clots scared people, but the clots were rare, affecting one in every million vaccine recipients, while the blood clots that can happen due to COVID-19 are significantly more common.
“The reason that we’re here as health professionals recommending the vaccine,” Prystowski said, “is that we feel in our risk calculation that it is so much safer to get the coronavirus vaccine than it is to risk the possibility of getting severe disease from coronavirus, which unfortunately has been really unpredictable.”
He said young people are the most at risk for getting a severe disease from COVID-19 because they are the least likely to be vaccinated.
Most of the 52 people in Sonoma County ICUs are in their 20s and 30s Jackson said; 92% are unvaccinated and 8% are partially vaccinated.
“So we want our younger people to know this is not an older person’s disease. Coronavirus is unfortunately indiscriminate. It has no prejudice against anyone, and young people are at great risk,” Jackson said.
She said getting vaccinated also helps protect family members who are too young to receive the vaccine themselves.
Q: What is the vaccine actually doing inside the body? Is it editing my genes?
Prystowsky said the vaccine uses mRNA, or ribonucleic acid, which is different from DNA, deoxyribonucleic acid, that makes up genes. The mRNA from the vaccine doesn’t enter the nucleus of the cell, where DNA resides, and so it never interacts with genes.
Also, there is no coronavirus in the vaccine, he said. When the vaccine is injected into the arm, it is picked up by the immune system’s first responders, called antigen presenting cells, which decide whether substances are OK to be in the body. Then mRNA from the vaccine enters the ribosomes of the antigen presenting cells, which are the cell factories for making proteins. The “m” in mRNA stands for messenger, and it works as an instruction manual for antigen presenting cells to make the spike protein that is found on the surface of coronavirus. Antigen presenting cells bring the spike protein to other cells in the immune system, which teaches the immune system to identify coronavirus without ever being exposed to it.
“So then if you get exposed to coronavirus, your immune system already knows to get rid of whatever is attached to that spike protein,” Prystowsky said. “Since your body is prepared, you are protected from getting as sick.”
Prystowsky said the mRNA disappears after the spike protein is created. RNA doesn’t survive long in the body, which is also why vaccines need to be frozen at very cold temperatures to keep them from thawing and losing function.
Prystowsky compared the spike protein to a hat that the coronavirus always wears.
“With the variants I sometimes talk about the hat having a different rim color. So it’s a little different, but your body still knows that hat really well,” Prystowsky said.
Q: Could getting the vaccine make a high-risk person more susceptible to developing Type 1 diabetes?
Prystowsky said he hadn’t heard of any such risk, but said that no one was excluded from the initial phase 3 trials because researchers wanted to see how well vaccines perform in people with chronic illness. To his knowledge, it works just as well with people with diabetes. However, people with an underlying chronic illness should consult their primary care physician to ask if they recommend the vaccine. He would guess the majority of doctors would say it’s safer to get the vaccine when you have chronic illness than suffer the possibility of getting sick with coronavirus.
Q: How can the average person trust medical professionals given they are trained to go along with their colleagues, and how can people of color trust medical professionals when doctors were historically involved in past systemic abuses? What about people of color who received poor treatment in the medical system because of their race?
“We have to acknowledge that there have been disparities in healthcare because individuals in certain areas in certain systems have been denied equal health care,” Jackson said.
While there have also been direct abuses on the basis of race, like the Tuskegee syphilis study, where people of color were treated as “guinea pigs” for medical experimentation, the case of the COVID-19 vaccine is completely different, she said.
“This is not a case where something harmful is being pushed upon the community of persons of color,” Jackson said. “Just the opposite. People who are not of color are the primary ones receiving the vaccine. So we need to have zero concerns along those lines.”
Baldwin said medical professionals follow the data instead of simply fist bumping and going along with each other.
“Most scientists and physicians look into research and data with a healthy dose of skepticism, which is the basis of science,” Baldwin said. “‘Prove to me what you’re saying is really true.’ Sometimes it doesn’t pan out. This time it has.”
Delashay Carmona Benson, president of SRJC’s Student Government Assembly and Black Student Union, asked how she could get her students and members of BSU to trust doctors and the government enough to get vaccinated.
Jackson said she should encourage people who have questions to participate in webinars and Q&A sessions like this one.
Prystowsky said if you want to make an impact with your family and friends, you should tell them your story, especially if you’re vaccinated.
Q: If a pregnant person got vaccinated, would the vaccine hurt the baby?
Prystowsky said it’s especially important for pregnant people to get vaccinated because one vaccination can benefit two people: the parent and the baby. There is a similar risk calculation with pregnancy.
“What’s riskier, not being vaccinated and catching a more contagious variant while you’re pregnant; or being vaccinated and knowing your body is able to mount some protection for you and the baby?” Prystowsky said.
Q: How do we know that big pharmacies aren’t making these vaccines and booster shots a cash cow by making COVID more serious than it might be?
“We have two processes that are parallel. One is this is a capitalistic society, and the companies that manufacture the vaccinations, Pfizer, Moderna and J&J, are for-profit,” Dr. Jackson said. “So they are seeking to make an income on the products they produce. That’s not illegal and it’s not unethical. It’s a part of the system.”
However, she said we also have checks and balances. The CDC gives recommendations on what is needed medically, and when a company makes a product to meet that need, the FDA looks at their product to make sure it is safe and effective.
“So even though a company wants to make money on their vaccinations, they can not do so if it’s not recommended as a valid therapy, and it’s not approved by the FDA,” she said.
Dr. Baldwin said a perfect example is when Pfizer applied for widespread booster vaccinations for all adults, and the FDA didn’t follow that recommendation because FDA officials didn’t see evidence that it was needed right now.
Q: If the FDA says this is safe and effective to use as a booster, does that mean we all should take it?
“The question about a booster is when do you need or if you need a booster? Who are the people who need some benefit from an extra challenge to your immune system?” Prystowsky said.
Since the vast majority of people in hospitals are unvaccinated, the vaccination is still effective enough to prevent serious disease, he said. As scientists, they are waiting on the checks and balances of the CDC and FDA to determine when boosters are a good idea and for whom. So far, the FDA has recommended boosters for people 65 and older.
Dr. Norwick said if students are ready to be vaccinated or have more questions about the vaccine, they can call Student Health Services at 707-527-4445 for an appointment on the Santa Rosa or Petaluma campus.